CYTOPOINT® is an injection that controls itch for 4 to 8 weeks*
CYTOPOINT is a safe, effective, long-lasting treatment to help control itch due to atopic dermatitis. It is an injection that is given by your veterinarian that targets itch at its source.
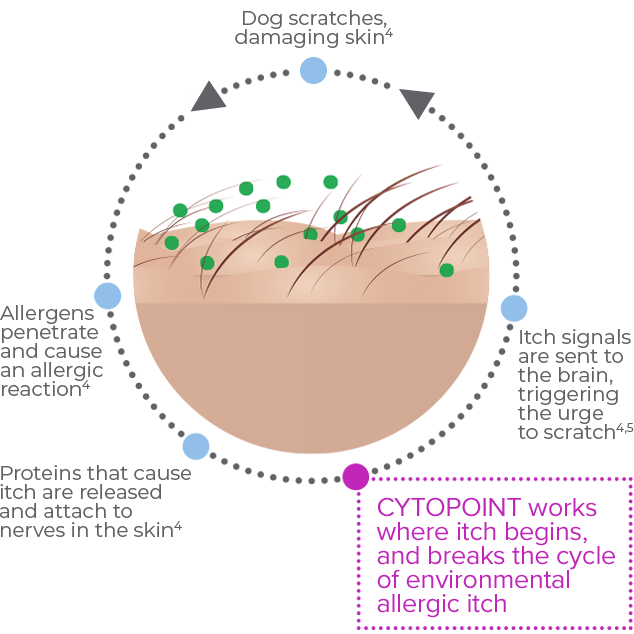
CYTOPOINT works like your dog’s own immune system. It is specifically designed to target and neutralize one of the main proteins that send itch signals to your dog’s brain that triggers scratching, licking, and chewing. CYTOPOINT blocks signals that trigger environmental allergic itch, so the constant scratching can stop so the skin has a chance to heal. In fact, in a study, CYTOPOINT helped damaged skin begin to heal within 7 days.1
Relief of environmental allergic itch at long last!
In studies, after one injection, CYTOPOINT started controlling itch within 1 day and provided relief that lasts for 4 to 8 weeks.*1 That means your dog may be able to go as long as 8 weeks1 between injections.


How does CYTOPOINT work?
CYTOPOINT is not like traditional pharmaceutical treatments for environmental allergic dog itch. This biological medication is a protein, not a drug, that works like your dog’s own immune system to block the main protein that sends signals that trigger itch.2,3
The itch cycle in dogs with environmental allergic itch
How will you know when it’s time for another CYTOPOINT injection?
You should schedule a progress exam with your veterinarian 4 weeks after the initial injection. However, every dog is different, so monitoring your dog’s scratching behaviour is important. If you see that the itch is acting up before the progress exam, call your veterinarian—your dog’s injection schedule may need to be adjusted.
The CYTOPOINT Environmental Allergic Itch Tracker can help you keep track of your dog’s itch. It’s simple to use and downloadable. Remember to take the tracker to your progress exam, so you can discuss the information with your veterinarian.
CYTOPOINT is safe for dogs of all ages
CYTOPOINT is safe to use in dogs of any age. It can also be used in dogs on many other commonly used medications, or dogs with other diseases.6 In fact, a clinical study showed dogs receiving CYTOPOINT had no more side effects than dogs receiving placebo (injections without medication).6 And since CYTOPOINT is not a drug-based treatment, it does not put pressure on the liver and kidneys.**
With CYTOPOINT, you gain the comfort of knowing every injection is delivered effectively and safely during an office visit. Your veterinarian administers a complete dose that helps relieve itch for up to 8 weeks,* making the treatment of this environmental allergic skin condition easier for you to manage.
How long will my dog need CYTOPOINT treatment?
Some dogs have year-round disease and may need continuous treatment with CYTOPOINT, whereas other dogs may only need CYTOPOINT seasonally. You and your veterinarian should decide together what is best for your dog’s specific situation.
*Repeat administration every 4 to 8 weeks as needed in the individual patient.1
**As with any other treatment, it is good medical practice for the veterinarian to make an appropriate risk/benefit assessment for each individual patient when considering CYTOPOINT. Please contact your veterinarian if you would like to know more information or have questions about any potential adverse effects for CYTOPOINT.
References: 1. Company Data, Study No. C863R-US-12-018, Zoetis Inc. 2. Gonzales AJ, Humphrey WR, Messamore JE, et al. Interleukin-31: its role in canine pruritus and naturally occurring canine atopic dermatitis. Vet Dermatol. 2013;24(1):48-53. doi:10.1111/j.136S-3164.2012.01098.x. 3. Olivry T, Bäumer W. Atopic itch in dogs: pharmacology and modeling. In: Cowan A, Yosipovitch G, eds. Pharmacology of Itch, Handbook of Experimental Pharmacology. 2015:357-369. 4. Marsella R, Sousa CA, Gonzales AJ, et al. Current understanding of the pathophysiologic mechanisms of canine atopic dermatitis. J Am Vet Med Assoc. 2012;241(2):194-207. doi:10.2460/javma.241.2.194. 5. Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet Dermatol. 2010;21(3):233-248. doi:10.1111/j.1365-3164.2010.00889.x. 6. Company Data, Study No. C961R-US-13-051, Zoetis Inc.
The product information provided in this site is intended only for residents of Canada. The products discussed herein may not have marketing authorization or may have different product labeling in different countries. The animal health information contained herein is provided for educational purposes only and is not intended to replace discussions with an animal healthcare professional. All decisions regarding the care of a veterinary patient must be made with an animal healthcare professional, considering the unique characteristics of the patient.
Zoetis® and Cytopoint are registered trademarks of Zoetis or its licensors, used under license by Zoetis Canada Inc.
© 2020 Zoetis Services LLC. All rights reserved.

 Learn about environmental dog itch and treatment
Learn about environmental dog itch and treatment 